Pyrene–Actin Polymerization Assay: A Review
The pyrene–actin polymerization assay is a cornerstone method for studying actin dynamics in vitro. It provides a sensitive and quantitative way to monitor the assembly of actin filaments, helping researchers understand how actin-binding proteins and small molecules influence filament formation, elongation, and stability.
Actin labelled with pyrene available to buy:
Principle
Actin is a highly dynamic protein that exists in two forms: the monomeric (G-actin) and filamentous (F-actin) states. The transition between these states drives many cellular processes, including motility, division, and shape maintenance. To study this process, scientists often use actin labelled with pyrene—a fluorescent probe that increases its emission intensity when actin polymerizes. This fluorescence change provides a direct readout of filament formation over time.
When actin monomers begin to assemble, pyrene fluorescence rises steadily until the reaction reaches equilibrium, where the rates of polymerization and depolymerisation balance. By tracking fluorescence over time, one can visualise and quantify the kinetics of nucleation (the lag phase), elongation (the rapid growth phase), and steady-state (the plateau phase). This makes the assay an elegant tool for dissecting how various factors modulate actin assembly.
Advantages and Applications
The pyrene–actin assay is valued for its simplicity, reproducibility, and quantitative nature. It requires relatively small amounts of protein and can be performed in standard cuvettes or 96-well fluorescence plates. Because the signal directly reflects filament formation, the assay allows precise comparisons of polymerization rates under different experimental conditions.
This approach has been instrumental in studying the effects of actin-binding proteins such as profilin, cofilin, and formins, as well as complex systems like the Arp2/3-mediated branching pathway. It has also been widely used to assess how mutations or post-translational modifications (such as acetylation or arginylation) alter the intrinsic dynamics of actin.
Beyond basic research, pyrene–actin assays provide valuable insights in pharmacological studies. Inhibitors like latrunculin, which prevents actin polymerization, or phalloidin, which stabilises filaments, can be evaluated for potency and mechanism using the same approach. This versatility has made the assay a gold standard for biochemical investigations of actin behaviour.
Typical Workflow
Although the assay’s exact setup can vary, it typically involves mixing monomeric actin (partially labelled with pyrene) in a buffer that promotes polymerization. The reaction is then monitored continuously using a fluorescence reader with excitation near 365–370 nm and emission near 407–410 nm. The resulting fluorescence curve reveals key kinetic parameters such as the lag time before filament growth begins and the apparent polymerization rate during elongation.
Researchers often normalise fluorescence signals to compare different samples, expressing data as the fraction of actin polymerized. By analysing several actin concentrations, it is also possible to determine the critical concentration—the minimal monomer level required for polymerization.
Interpretation and Insights
The shape of the fluorescence curve tells a story about how actin behaves under given conditions. A longer lag phase suggests slower nucleation, perhaps due to inhibitory proteins or missing cofactors. A steeper slope indicates faster elongation, often promoted by nucleators or processive elongation factors. The plateau height reflects the final amount of F-actin formed, which relates to both monomer availability and the equilibrium constant for polymerization.
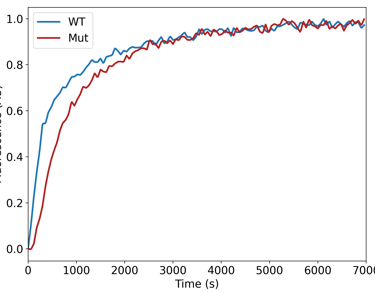
Comparative studies—such as those examining actin from different species, tissues, or modification states—can reveal subtle but biologically important differences in filament dynamics. For example, acetylated and arginylated actin variants have been shown to display distinct polymerization behaviours that correlate with their cellular functions.
Broader Context
Since its introduction in the 1980s, the pyrene–actin assay has remained a workhorse of actin biochemistry. Despite newer imaging and single-molecule techniques, it continues to offer a robust, quantitative foundation for understanding actin assembly in solution. When combined with complementary methods—such as TIRF microscopy, sedimentation assays, or structural studies—the pyrene–actin assay provides a complete picture of the molecular choreography underlying cytoskeletal dynamics.
Conclusion
The pyrene–actin polymerization assay elegantly bridges biochemistry and cell biology by translating actin’s invisible molecular motions into measurable fluorescence signals. Its enduring relevance lies in its precision, adaptability, and conceptual clarity. Whether used to explore fundamental mechanisms of actin regulation or to evaluate the effects of mutations and drugs, this classic assay remains one of the most powerful tools available for studying the life and dynamics of actin filaments.